A year has passed since the first case of COVID-19 was reported in Singapore. Ensuring a strong testing capability continues to be one of the nation’s key strategies to contain the spread of the stealthy virus, as large-scale testing allows for the early detection and isolation of cases before they infect others.
Within a year, Singapore significantly scaled up its daily testing capacity from an average of 2,900 tests in the initial days of the outbreak to 40,000 tests by year end. As of 1 February 2021, more than 6.5 million tests have been conducted for the stealthy coronavirus, according to the Ministry of Health.
Two homegrown COVID-19 test kits – the Fortitude and Resolute kits distributed by local firms MiRXES and Advanced MedTech Holdings (AMTH) respectively – contributed to the acceleration efforts. The kits have been deployed in the hospitals and labs in Singapore. To date, both firms have distributed a total of more than seven million kits locally and globally.

The Fortitude (right) and Resolute kits
The Fortitude Kit was the first “ready-made” COVID-19 hospital lab diagnostic test kit approved by the Health Sciences Authority (HSA) for clinical use in Singapore. Launched in February 2020, within a month of the virus sequence’s publication online, Fortitude can accurately detect the presence of the SARS-CoV-2 virus using the real time RT-PCR (reverse transcription-polymerase chain reaction) method.
To complement the other diagnostics available in the local market, DSO Laboratories and A*STAR co-developed and launched the Resolute kit in July, building on the innovative molecular-based assay that DSO successfully developed four months earlier. Unlike conventional PCR test kits, Resolute is a direct-PCR test that eliminates the need for the extraction of viral RNA from patient test samples, thereby minimising potential human errors and halving the time required to complete the test.
The speed at which the new diagnostics innovations were translated into clinically validated mass-manufactured kits was thanks to the public-private partnership between the R&D ecosystem and the medical technology manufacturers.
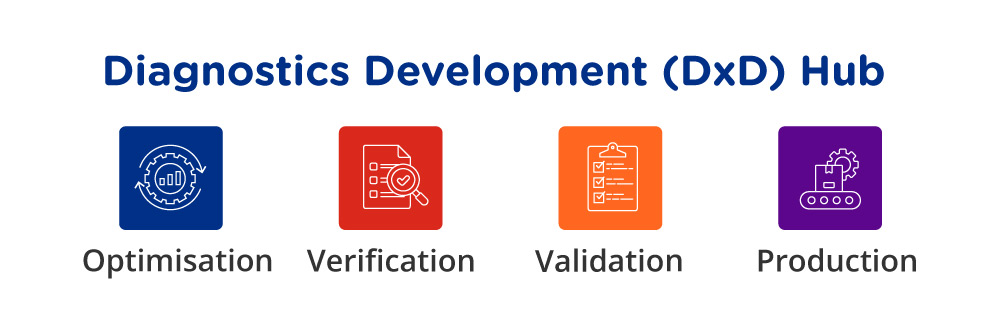
The Diagnostics Development (DxD), a national platform funded by National Research Foundation and led by A*STAR, was roped in to accelerate the speed at which local companies translated the two new COVID-19 tests into market-ready diagnostic solutions. As a national platform, DxD Hub brought together key stakeholders, from clinicians and researchers to entrepreneurs and industry professionals, enabling companies to benefit from a cross-pollination of ideas and knowledge.
When the time came for starting the mass production of the kits, DxD Hub evaluated potential manufacturers through a rigorous evaluation process, and decided to work with local small and mid-sized enterprises, MiRXES and AMTH. DxD Hub then helped enhance their capabilities, including in product development and manufacturing processes, to accelerate the scale-up activities.
MiRXES was an ideal candidate for taking the baton over from DxD Hub. An A*STAR spinoff, it already had a working relationship with DxD Hub since 2014 in developing different diagnostics. Its experience producing another PCR-based diagnostics – GASTROClear, a blood test for early stomach cancer detection – made it easier for them to get started on the urgent task of mass-producing the Fortitude Kit. MiRXES also quickly scaled the delivery of the kit, which received the CE Mark and regulatory approval in Philippines, Panama, Peru, Colombia, and Honduras, to more than 45 countries globally.
On the other hand, AMTH, an established medical device company and wholly-owned subsidiary of Temasek, brought its manufacturing expertise to bear on the task of significantly scaling up Singapore’s testing capability. It set up one of the largest automated in-vitro diagnostics manufacturing facilities in the country in just six weeks, with the capacity to produce two million Resolute tests per month.
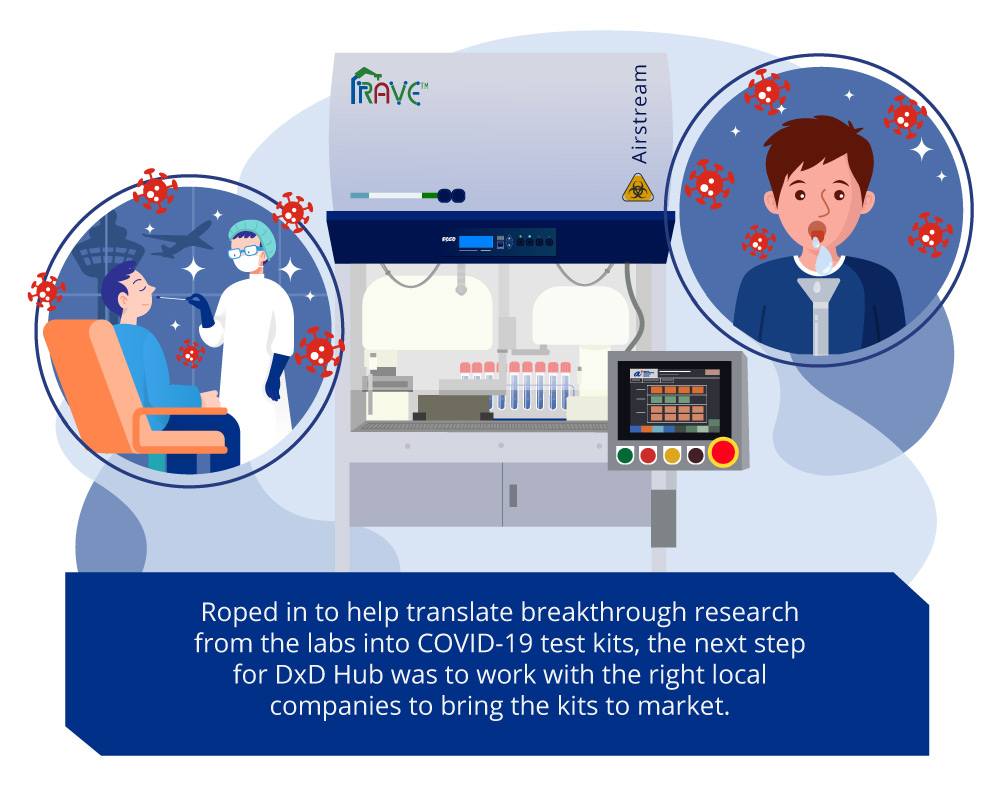
AMTH also distributes the Rapid Automated Volume Enhancer (RAVE), a robotics lab system co-developed by A*STAR’s Advanced Remanufacturing and Technology Centre (ARTC), the Singapore Institute of Manufacturing Technology (SIMTech) and DxD Hub. By reducing the manual steps in the preparation of a test assay such as unscrewing tubes and pipetting, RAVE can expedite the Resolute testing process and handle a throughput of around 4,000 samples a day. The robotics system also reduces test contamination and infection risks for laboratory workers.

Even after the successful launch of the two kits, DxD Hub continues to work with its collaborators to develop improvements for them as the pandemic situation evolves.
DxD Hub and TTSH developed an expanded Fortitude Kit that can test for COVID-19 as well as influenza A and B. As patients with COVID-19 or flu exhibit a set of similar symptoms, it is difficult for healthcare professionals to differentiate between the two. This new kit allows for a more accurate and timely diagnosis.
As for Resolute , DxD Hub and DSO made it work with deep throat saliva and throat samples, on top of the conventional nasal swab samples. Saliva sample collection is much less invasive and more comfortable for patients than having swabs inserted into the back of the nose or throat. It also makes the test easier to administer and allows patients to provide the samples themselves without the need for swabbers, mitigating the risks of potential virus spread.
Dr Sidney Yee, Chief Executive Officer of DxD Hub, said: “It has been a challenging but rewarding and worthwhile endeavour for DxD Hub to quickly facilitate the knowledge and technology transfer to the local medtech companies for the Fortitude and Resolute kits, aiding them from product design, validation to mass production.”
“As we look ahead to prepare ourselves for the next pandemic, we will continue to expand our productisation capabilities to serve the ecosystem more comprehensively,” she added.