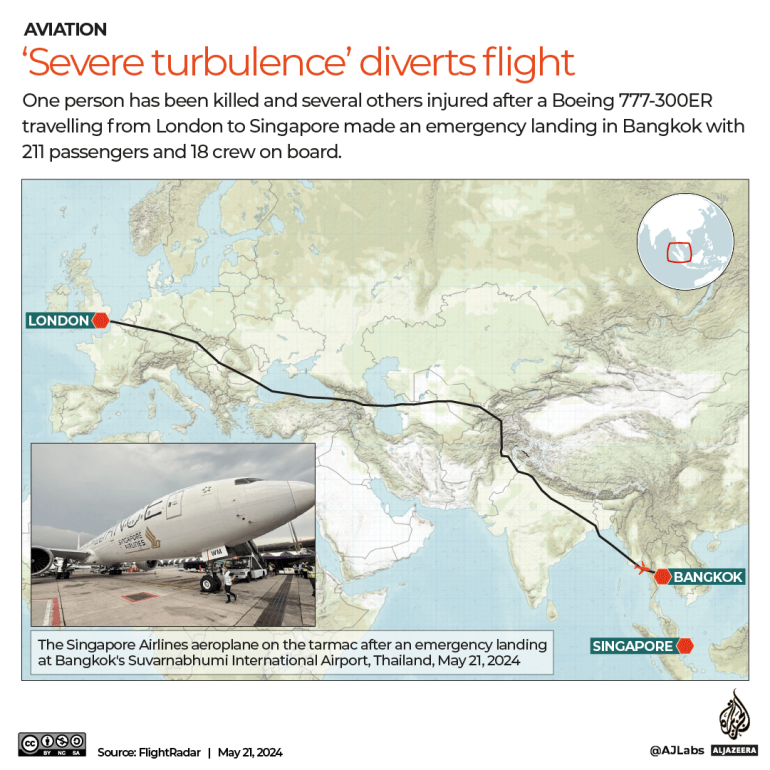
A test kit that can accurately identify SARS-CoV-2 antibodies in 15 minutes and employs a lateral flow format, similar to those used in home pregnancy tests, has been co-developed between A*STAR and diagnostics company MP Biomedicals.
Serological test kits are especially important for contact-tracing purposes given that there are large numbers of asymptomatic COVID-19 cases around the world. The ASSURE® kit can determine if one has acquired immunity in the form of antibodies generated by the human body after exposure to the SARS-CoV-2 virus.
Easy to administer by healthcare professionals at the point of care, ASSURE® detects immunoglobulin (Ig) antibodies – IgM and IgG – produced by the human immune system in response to exposure to SARS-CoV-2. Studies have shown that levels of IgM and IgG appear to be correlated with the severity of COVID-19, thus making them good biomarkers for confirming prior COVID-19 infections.
While antibody tests should not be used in the clinical diagnosis of COVID-19 infections, especially within the first 14 days of illness, they can be helpful for identifying asymptomatic individuals or those with only mild symptoms who were not subjected to RT-PCR testing.
With its specialty in infectious disease testing development and manufacturing, MP Biomedicals will be manufacturing ASSURE locally.
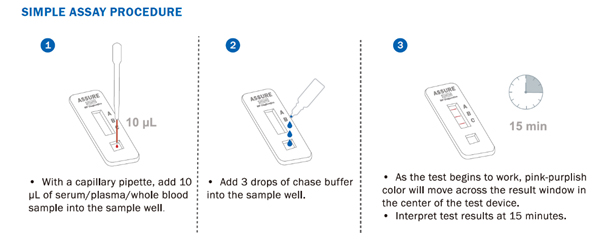
The development of ASSURE® was part of concerted efforts by Singapore’s R&D and clinical ecosystem, both public and private, to develop diagnostics, therapeutics, and preventative tools in the fight against the COVID-19 pandemic.
The technology behind ASSURE® relies on proprietary synthetic SARS-CoV-2 proteins identified by scientists from A*STAR’s Institute of Molecular & Cell Biology (IMCB), led by Associate Professor Tan Yee Joo, Joint Senior Principal Investigator at IMCB and National University of Singapore’s Yong Loo Lin School of Medicine.
MP Biomedicals then used this technology to develop ASSURE® based on their lateral flow platform. The Diagnostics Development (DxD) Hub, a national platform hosted by A*STAR’s commercialisation arm, A*ccelerate, co-developed the validation protocols and quality controls.
The National University Hospital’s (NUH) Department of Laboratory Medicine evaluated the test kit, which demonstrated good results for both serum and whole blood. The sensitivity of the kit performed well as compared to commercial immunoassays, when tested with convalescent blood from recovered COVID-19 patients in the clinic.

ASSURE® has been granted Provisional Authorisation by the Health Sciences Authority (HSA) for its intended use in Singapore. It has also been distributed to regions such as Europe, Africa and South America. MP Biomedicals intends to file for Emergency Use Authorisation (EUA) from the US FDA as well.
“The development and manufacture of ASSURE® is a successful collaboration between MP Biomedicals and A*STAR through tremendous joint work,” said Dr Delynn Xu, Senior R&D Manager at MP Biomedicals. “We are not the first one in the market but chasing the best performance is always our primary goal. With this rapid antibody test kit, we are proud to contribute to the global fight against COVID-19.”
“It is absolutely critical that we continue to transfer R&D know-how to biotech companies, to scale up and let more labs in Singapore tap on this diagnostics test kit to screen patients,” said Dr Sidney Yee, Chief Executive Officer of DxD Hub. “This rapid serological point-of-care test kit for COVID-19 by MP Biomedicals and A*STAR will complement global efforts to develop more efficient diagnostics, as the COVID-19 situation continues to evolve.